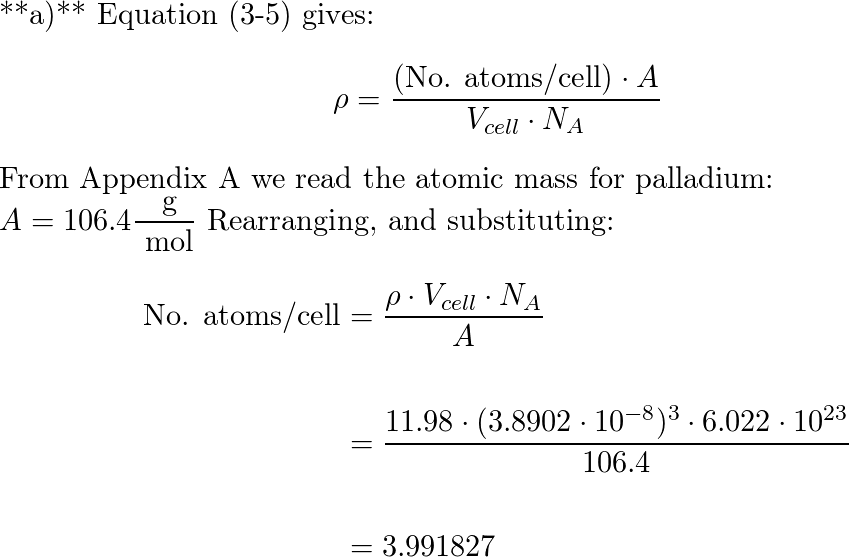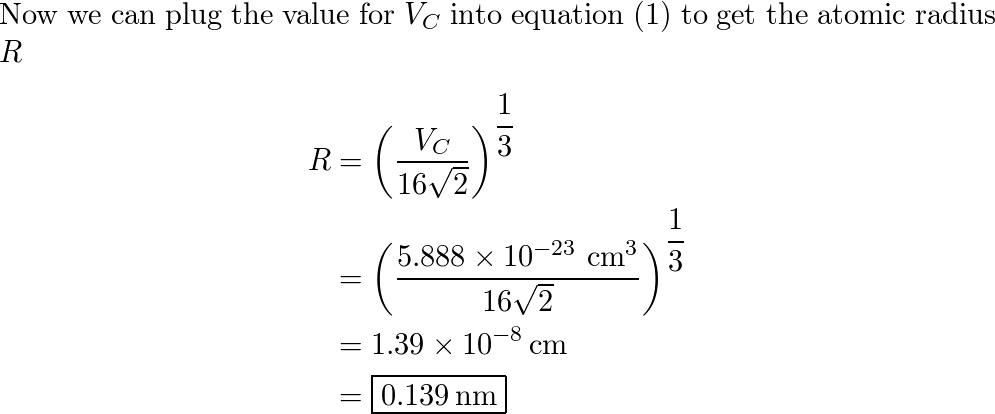0.25 Gram Solid Palladium Metal Pellet - Pure Element 46 Sample - Free Shipping: Amazon.com: Industrial & Scientific

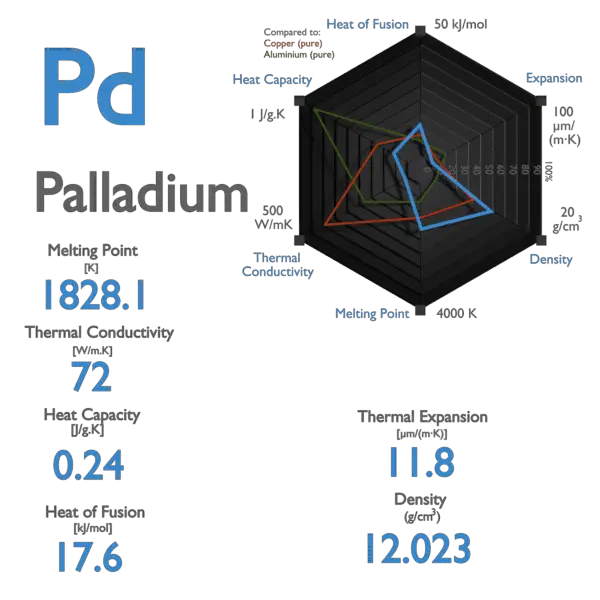
the density of palladium is 12.0g/Cm^3. what volume in liters would be occupied by 532 g of palladium? - Brainly.com

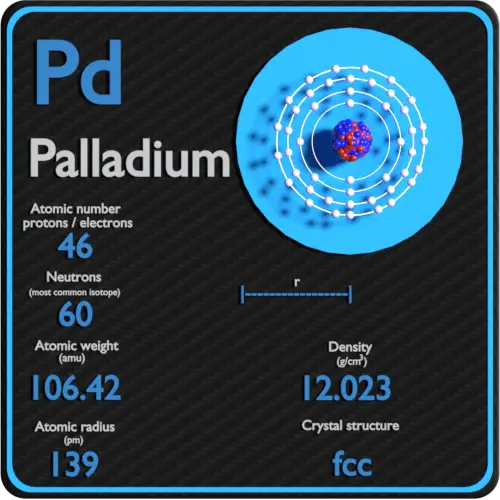
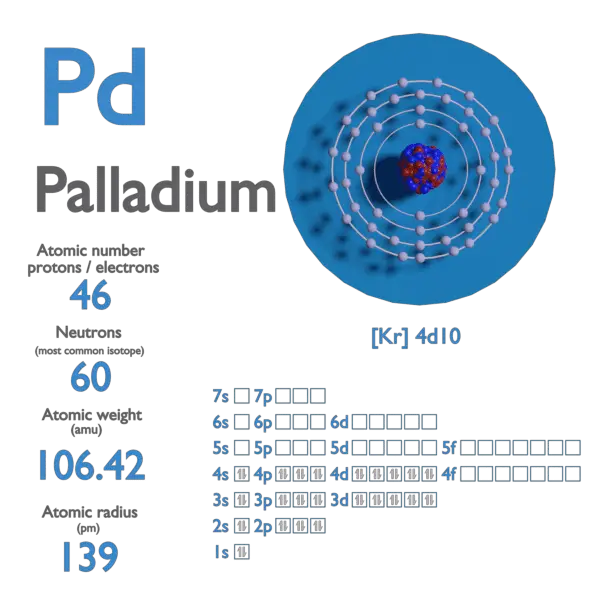
SOLVED: Calculate the radius of a palladium atom, given that Pd has an FCC crystal structure, a density of 12.0 g/cm3 , and an atomic weight of 106.4 g /mol.

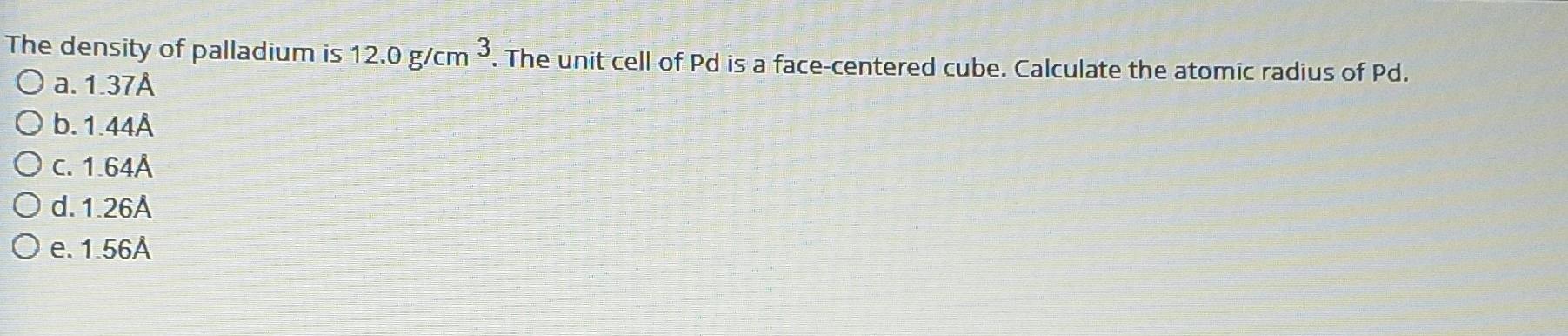
SOLVED: Calculate the radius of a palladium atom in nm, given that Pd has an FCC crystal structure, a density of 12.0 g/cm3 and an atomic weight of 106.4 g/mol. A. 138

Exam 1 Review Solutions.docx - Exam 1 Review 1. Calculate the radius of a palladium Pd atom given that Pd has an FCC crystal structure a density of | Course Hero